class 7 Science Chapter 5 Quick Revision Notes
Class 7 science chapter 5 quick revision notes (Acid Base And Salt):
This study guide, which includes important notes for Class 7 Science Chapter 5 on Acids, Bases, and Salts, will aid you in getting ready for your exams. Class 7 science chapter 5 quick revision notes is developed by Web Tutors Point that clearly explain all the key points and ideas to help you comprehend the material better.
Types of substances based on their chemical nature.
Substances can be divided into acids, bases, and salts according to their chemical nature.
Describe ACIDS.
Everything with a sour flavour contains acids. things like lemon, vinegar, and tamarind. Compounds called acids always contain the hydrogen element as one of the constituent parts of the acid molecule. Hydrochloric acid (HCl), as an example of acid.
The term “acidic substance” refers to substances that include acids.
Various acid types based on sources they are obtained from:
How many different kinds of acids are there?
Acids come in two different varieties depending on where they come from: Mineral acids and organic acids.
What are Minerals?
Minerals are naturally occurring inorganic (compounds that do not include carbon) solids.
Describe mineral acids.
Acids that are created from the minerals found in the ground are known as mineral acids. For instance, Sulfuric acid (H2SO4), Hydrochloric acid (HCL), also referred to as the King of Chemicals and frequently utilised, etc. They are powerful acids. Strong acids are capable of fully splitting in solution.
Acids are divided into concentrated and diluted acids based on how much water they contain.
What is concentrated acid?
Acid that contain low or small quantity of water called concentrated acids.
What is diluted acids?
Acids of low concentration or acids that contain more water than concentrated acids are referred to as diluted acids. They can obtained by adding water to a concentrated acids.
What are Organic Acids?
Organic Acids are naturally occurring acids that can be found in both plants and animals. These acids are weak. Acids that do not entirely split in solution are considered weak acids.
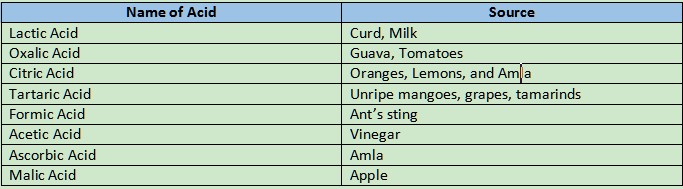
Why Red ants and bee stings deliver a sharp pain?
Red ants and bee stings both contain Formic acid, which delivers a sharp pain.
Acids’ characteristics:
- Sour flavour.
- Blue Litmus is turned red by acids.
- Soluble in water.
Why do we keep acids in glass rather than metal?
Acids have the potential to damage metals like iron and Aluminium. Because of this, acids are kept in glass containers rather than metal ones.
Uses of Acids
Acids are frequently utilized in industries and can be found in common goods. Our stomachs contain hydrochloric acid, which aids in the food digestion process. The following table lists the uses for various acids;
Acids | Uses |
Hydrochloric acid | Remove dust from metals |
Sulphuric acid | Used in batteries, paints |
Nitric acid | Explosive, dyes, plastic |
BASES
Bases are compounds possessing either oxygen or oxygen and hydrogen. A base that only contains oxygen is referred to as an oxide, while a base that also contains hydrogen and oxygen is referred to as a hydroxide.
Basic substances are those that have bases in them.
Types of Bases based on reaction:
There are two types of bases: strong bases and weak bases, which differ in how they contribute to a reaction.
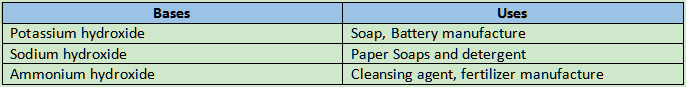
Strong bases: A few of the bases have corrosive properties and could cause skin burns. Strong bases are those types of bases. Sodium hydroxide, calcium hydroxide, and potassium hydroxide are a few examples.
Weak bases: A few of the bases are not naturally corrosive. Weak bases are those types of bases. Examples are copper hydroxide, ammonium hydroxide, and magnesium hydroxide.
Characteristics of bases:
A bitter flavour.
Bases turn blue on the litmus test.
Some bases are known as Alkalis because they are soluble in water (potassium hydroxide and sodium hydroxide).
In a solution, blue litmus paper is immersed. It’s still blue. What type of solution is being offered? Explain.
We are aware that acids cause blue litmus paper to turn red. A solution cannot be acidic if a blue litmus paper is dipped in it and it does not turn red. Therefore, the answer can be either basic or neutral.
Is the distilled water neutral, acidic, or basic? How might you validate it?
The chemical makeup of distilled water is neutral. We can use litmus paper to check it. When distilled water is dropped in little amounts onto red/blue litmus paper, the colour doesn’t change.
SALT
What is salt?
Salt and water are produced when an acid and a base are combined.
A salt can be basic, neutral, or acidic.
Acidic salts: Acidic salts are created when Strong acids and Week bases combine. The pH (A measure of the concentration of hydrogen ions, the acidity or alkalinity of a solution. The pH-scale is normally between 0 and 14.) of these salts is lower than 7. Examples are Aluminum chloride, and ammonium chloride.
Basic salts: When strong bases and weak acids react, basic salts are created. The pH of these salts is greater than 7. For instance, sodium acetate and sodium carbonate.
Neutral salts: Strong acids and strong bases react to generate neutral salts. The pH of these salts is 7. For instance, potassium nitrate and sodium chloride (NaCl).
Chemical Properties of Salts
The majority of salts are soluble in water, according to their chemical properties.
Water containing salt solutions acts as a good conductor of electricity.
While some salts are crystallised in white, others come in different colours. For instance, ferrous sulphate is green and copper sulphate is blue.
Some salts have water molecules caught inside of them; this is known as crystallisation water. Hydrated salts are salts that have crystallised water in them. Blue vitriol, as an illustration.
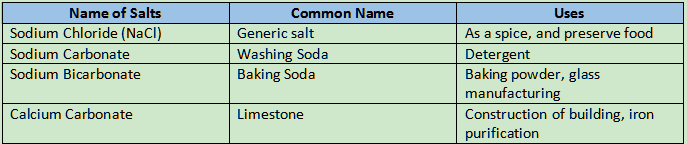
Uses of salts
Neutralization Reaction
What is neutralization?
A reaction between an acid and a base that produces salt and water is known as neutralization.
Uses of Neutralization Reactions:
Indigestion Treatment: The stomach produces too much acid, resulting in indigestion. By consuming an antacid like milk of magnesia, which improves the situation, it neutralizes the acids.
Ant Sting Treatment: An ant injects formic acid into the skin when it bites. The next step is to neutralize the sting by applying moist baking soda, also known as sodium bicarbonate, or Calamine, which includes zinc carbonate, to the affected area.
Soil Treatment: The addition of quicklime (calcium oxide) or slaked lime (calcium hydroxide) to an overly acidic soil will neutralize it.
Dental protection: Toothpaste is one of the best frequently used methods for neutralizing acids. The alkali in toothpastes neutralizes the weak acid that bacteria make, protecting teeth from harm.
Any substance that has neither an acidic nor a basic character is considered a neutral substance.
Prior to being dumped into water bodies, factory waste is neutralized.
Ans: Factory waste is neutralized before being dumped into water bodies since excess acids or bases can be harmful and even lethal to aquatic life.
Indicators
We can’t taste everything to determine what it is. As a result, we employ indicators.
An indication is something that can tell whether something else is basic or acidic in nature.
By altering their colour, the indicators reveal the presence of an acid or base in a sample. Turmeric, China rose petals, and Litmus, for instance, are some examples of natural indicators. Natural indicators are those that can be found in nature.
Litmus is a naturally occurring indicator made from lichens. There are paper strips and solution forms of Litmus (red litmus and blue litmus paper).
Lichens are the source of litmus. It is typically offered as a solution or in the shape of thin paper strips. The colour of blue litmus turns red when acid is applied. Red litmus paper turns blue when bases are added, changing its hue.
The natural indicator turmeric, which is yellow in neutral and acidic solutions, turns red when it comes into touch with alkaline solutions.
Additional Indicators:
Methyl Orange, for example, turns pinkish-red in acidic solutions while turning yellow in bases.
Phenolphthalein serves as an indication of acidity and basicity. In acidic solutions, it doesn’t change colour, but in alkali solutions, it turns pink.
About Acids, Bases, and Salts: Class 7 Science Chapter Notes
Students in Class 7 may find the chapter overwhelming, but once the principles are adequately explained, studying it will be much easier. You may find the fundamental definitions and explanations of the terms used in the Acids Bases and Salts Class 7 notes.
